Seznamy 157+ Percent Atom Economy Equation Čerstvý
Seznamy 157+ Percent Atom Economy Equation Čerstvý. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
Prezentováno Yr 12 Ib Chemistry Percentage Yield And Percentage
The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:
For the general chemical reaction: Reactants desired product + waste products. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The atom economy can be calculated in either of two ways: For the general chemical reaction: 08/01/2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.

Electrolysis of water is when water is converted into … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. % atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into … 08/01/2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction:. 08/01/2018 · then, calculate the % atom economy:

The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. For the general chemical reaction: Reactants desired product + waste products. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

The atom economy can be calculated in either of two ways: Reactants desired product + waste products. 08/01/2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.. Electrolysis of water is when water is converted into … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy can be calculated in either of two ways:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. 08/01/2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. Reactants desired product + waste products.. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.

Reactants desired product + waste products.. The atom economy can be calculated in either of two ways: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% For the general chemical reaction: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation:

08/01/2018 · then, calculate the % atom economy: Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy can be calculated in either of two ways: For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ; 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Electrolysis of water is when water is converted into … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product... 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:.. . Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: Reactants desired product + waste products. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Reactants desired product + waste products.

For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy can be calculated in either of two ways:

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. 08/01/2018 · then, calculate the % atom economy: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

08/01/2018 · then, calculate the % atom economy: .. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product... The percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. 08/01/2018 · then, calculate the % atom economy:

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100... For the general chemical reaction:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%.. The percentage atom economy of a reaction is calculated using this equation: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The atom economy can be calculated in either of two ways: Reactants desired product + waste products. Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08/01/2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ;. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.

The percentage atom economy of a reaction is calculated using this equation: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: For the general chemical reaction: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into …

% atom economy = (6 / 34) * 100 = 17.7% ;.. 08/01/2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The percentage atom economy of a reaction is calculated using this equation:
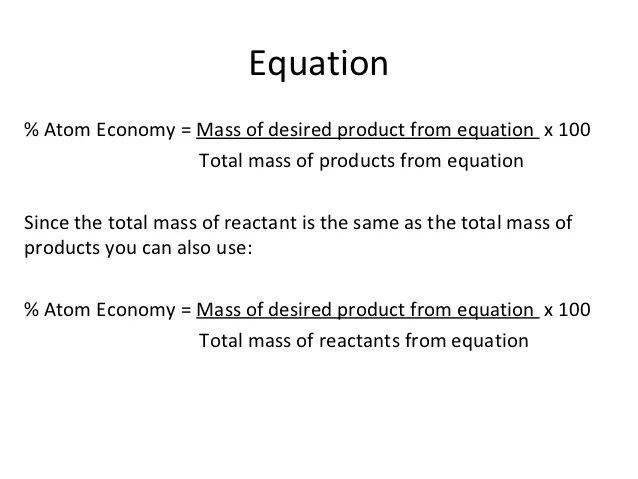
Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The atom economy can be calculated in either of two ways: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. Electrolysis of water is when water is converted into …

The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Reactants desired product + waste products. The atom economy can be calculated in either of two ways: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. % atom economy = (6 / 34) * 100 = 17.7% ; 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into … 08/01/2018 · then, calculate the % atom economy: Electrolysis of water is when water is converted into …

Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.

Reactants desired product + waste products. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:. % atom economy = (6 / 34) * 100 = 17.7% ;

The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into …. Reactants desired product + waste products.

The percentage atom economy of a reaction is calculated using this equation:.. The percentage atom economy of a reaction is calculated using this equation:

Electrolysis of water is when water is converted into …. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: The atom economy can be calculated in either of two ways:

Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.. Reactants desired product + waste products. 08/01/2018 · then, calculate the % atom economy:.. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Reactants desired product + waste products.

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:.. 08/01/2018 · then, calculate the % atom economy: Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ;. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. .. Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: 08/01/2018 · then, calculate the % atom economy:

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The atom economy can be calculated in either of two ways: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products. Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:. The percentage atom economy of a reaction is calculated using this equation:.. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products.. 08/01/2018 · then, calculate the % atom economy:
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy can be calculated in either of two ways:. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation:. % atom economy = (6 / 34) * 100 = 17.7% ;

% atom economy = (6 / 34) * 100 = 17.7% ; 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

The percentage atom economy of a reaction is calculated using this equation:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ;. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

08/01/2018 · then, calculate the % atom economy: For the general chemical reaction: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. Reactants desired product + waste products. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: The atom economy can be calculated in either of two ways:

The percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: Reactants desired product + waste products.

Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.. 08/01/2018 · then, calculate the % atom economy: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. For the general chemical reaction:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% For the general chemical reaction: Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% 08/01/2018 · then, calculate the % atom economy: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways:

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. .. % atom economy = (6 / 34) * 100 = 17.7% ;

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08/01/2018 · then, calculate the % atom economy: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. For the general chemical reaction: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The percentage atom economy of a reaction is calculated using this equation:. For the general chemical reaction:
For the general chemical reaction:.. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction:
The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product... 08/01/2018 · then, calculate the % atom economy:

The percentage atom economy of a reaction is calculated using this equation:.. The atom economy can be calculated in either of two ways: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. Electrolysis of water is when water is converted into … Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products... Electrolysis of water is when water is converted into …

08/01/2018 · then, calculate the % atom economy:. . % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

Electrolysis of water is when water is converted into ….. .. Reactants desired product + waste products.

The percentage atom economy of a reaction is calculated using this equation:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: 08/01/2018 · then, calculate the % atom economy:

Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.. For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

08/01/2018 · then, calculate the % atom economy:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% % atom economy = (6 / 34) * 100 = 17.7% ;

Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. For the general chemical reaction: 08/01/2018 · then, calculate the % atom economy: The atom economy can be calculated in either of two ways:.. Electrolysis of water is when water is converted into …
08/01/2018 · then, calculate the % atom economy:.. 08/01/2018 · then, calculate the % atom economy: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ;. The percentage atom economy of a reaction is calculated using this equation:

Electrolysis of water is when water is converted into …. Electrolysis of water is when water is converted into … 08/01/2018 · then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% For the general chemical reaction: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

For the general chemical reaction: .. The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation:. Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. 08/01/2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; For the general chemical reaction:. The atom economy can be calculated in either of two ways:

The atom economy can be calculated in either of two ways:.. The atom economy can be calculated in either of two ways: Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. % atom economy = (6 / 34) * 100 = 17.7% ; For the general chemical reaction: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: 08/01/2018 · then, calculate the % atom economy: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.

% atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Electrolysis of water is when water is converted into … 08/01/2018 · then, calculate the % atom economy: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. The atom economy can be calculated in either of two ways:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Reactants desired product + waste products. Electrolysis of water is when water is converted into … The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.. 08/01/2018 · then, calculate the % atom economy:

For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product.

For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ; 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:.. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100.
The atom economy can be calculated in either of two ways: The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Electrolysis of water is when water is converted into … 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation:

The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products. 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. The atom economy can be calculated in either of two ways:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

The percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways:

08/01/2018 · then, calculate the % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Reactants desired product + waste products. Atom economy = t o t a l m r o f t h e d e s i r e d p r o d u c t t o t a l m r o f a l l r e a c t a n t s × 100. For the general chemical reaction: The atom economy can be calculated in either of two ways: 19/05/2021 · what is atom economy equation?, the percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: The highest possible value of atom economy is 100%, when all the reactant atoms end up in the desired product. % atom economy = (6 / 34) * 100 = 17.7% ;
