Ideje 121+ Atom Diagram With Protons Neutrons And Electrons
Ideje 121+ Atom Diagram With Protons Neutrons And Electrons. Protons, neutrons and electrons of all the elements: Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Boron has 5 protons, 6 neutrons and 5 electrons: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Hydrogen has 1 proton, 0 neutron and 1 electron:
Prezentováno Basic Chemistry Tutorial 2 Drawing Atoms Learn Biology
20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Carbon has 6 protons, 6 neutrons and 6 electrons The mass of a proton is 1840 times greater than the mass of an electron.For each electron shell atom diagram, the element symbol is listed in the nucleus.
The atomic number of a sodium atom is 11 and its mass number is 23. Helium has 2 protons, 2 neutrons and 2 electrons: The nucleus is positively charged since the proton is positively charged and the neutron is neutral. • electrons have a negative charge. The mass of a proton is essentially the same as that of a neutron. • electrons surround the nucleus.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Lithium has 3 protons, 4 neutrons and 3 electrons: 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. • protons and neutrons are in the center of the atom, making up the nucleus. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. • ˚e charge on the proton and electron are. The nucleus of an atom contains protons and neutrons. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.
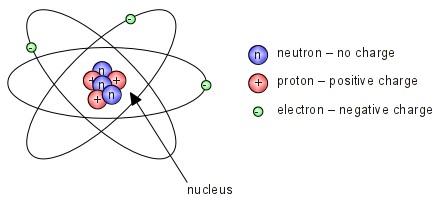
01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The atomic number of a sodium atom is 11 and its mass number is 23.. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.

Hydrogen has 1 proton, 0 neutron and 1 electron: • ˚e charge on the proton and electron are. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Protons, neutrons and electrons of all the elements: 112 zeilen · 01.11.2021 · atomic no. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.

• protons and neutrons are in the center of the atom, making up the nucleus. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. • protons and neutrons are in the center of the atom, making up the nucleus. Beryllium has 4 protons, 5 neutrons and 4 electrons: For that, we have electron shell diagrams. The nucleus of an atom contains protons and neutrons.

Boron has 5 protons, 6 neutrons and 5 electrons: • protons have a positive charge. Boron has 5 protons, 6 neutrons and 5 electrons: Beryllium has 4 protons, 5 neutrons and 4 electrons:.. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.

The atomic number of a sodium atom is 11 and its mass number is 23. • protons have a positive charge. Calculate the number of protons, neutrons and electrons it contains. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.

Protons, neutrons and electrons of all the elements: For each electron shell atom diagram, the element symbol is listed in the nucleus. The nucleus carries a positive electrical charge. • electrons surround the nucleus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The negative charge of one electron balances the positive charge of one proton… For that, we have electron shell diagrams.

• ˚e charge on the proton and electron are... Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. • protons and neutrons are in the center of the atom, making up the nucleus. The mass of a proton is essentially the same as that of a neutron. • electrons surround the nucleus. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: • protons have a positive charge. • ˚e charge on the proton and electron are. • electrons have a negative charge... Carbon has 6 protons, 6 neutrons and 6 electrons

06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: For each electron shell atom diagram, the element symbol is listed in the nucleus. • electrons have a negative charge. The mass of a proton is 1840 times greater than the mass of an electron. The nucleus is positively charged since the proton is positively charged and the neutron is neutral... Protons and neutrons reside in the nucleus and are together called nucleons.

The mass of a proton is 1840 times greater than the mass of an electron... • ˚e charge on the proton and electron are.. Beryllium has 4 protons, 5 neutrons and 4 electrons:

112 zeilen · 01.11.2021 · atomic no.. Hydrogen has 1 proton, 0 neutron and 1 electron: Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. • protons have a positive charge. The nucleus of an atom contains protons and neutrons. 112 zeilen · 01.11.2021 · atomic no. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:. Protons, neutrons and electrons of all the elements:
The mass of a proton is essentially the same as that of a neutron. Lithium has 3 protons, 4 neutrons and 3 electrons: 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. The atomic number of a sodium atom is 11 and its mass number is 23. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Carbon has 6 protons, 6 neutrons and 6 electrons • protons and neutrons are in the center of the atom, making up the nucleus. Calculate the number of protons, neutrons and electrons it contains. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms.

Beryllium has 4 protons, 5 neutrons and 4 electrons:. 112 zeilen · 01.11.2021 · atomic no. • protons have a positive charge. The atomic number of a sodium atom is 11 and its mass number is 23. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The nucleus of an atom contains protons and neutrons. For each electron shell atom diagram, the element symbol is listed in the nucleus... 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.
The nucleus carries a positive electrical charge.. The mass of a proton is 1840 times greater than the mass of an electron. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. • electrons surround the nucleus. The mass of a proton is essentially the same as that of a neutron. Protons and neutrons reside in the nucleus and are together called nucleons. The nucleus of an atom contains protons and neutrons. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

Helium has 2 protons, 2 neutrons and 2 electrons:.. Beryllium has 4 protons, 5 neutrons and 4 electrons: Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms... 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.

Protons and neutrons reside in the nucleus and are together called nucleons. Calculate the number of protons, neutrons and electrons it contains.. The mass of a proton is essentially the same as that of a neutron.

Carbon has 6 protons, 6 neutrons and 6 electrons. .. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons.

• electrons surround the nucleus. The mass of a proton is essentially the same as that of a neutron. 112 zeilen · 01.11.2021 · atomic no. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. For each electron shell atom diagram, the element symbol is listed in the nucleus. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The atomic number of a sodium atom is 11 and its mass number is 23. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

The nucleus carries a positive electrical charge. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. • protons and neutrons are in the center of the atom, making up the nucleus. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. For each electron shell atom diagram, the element symbol is listed in the nucleus. The atomic number of a sodium atom is 11 and its mass number is 23. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.. • protons and neutrons are in the center of the atom, making up the nucleus.

The mass of a proton is 1840 times greater than the mass of an electron. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The atomic number of a sodium atom is 11 and its mass number is 23. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.. Protons and neutrons reside in the nucleus and are together called nucleons.

For that, we have electron shell diagrams. • electrons surround the nucleus.

The nucleus is positively charged since the proton is positively charged and the neutron is neutral. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.

For each electron shell atom diagram, the element symbol is listed in the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Beryllium has 4 protons, 5 neutrons and 4 electrons: • ˚e charge on the proton and electron are. • protons and neutrons are in the center of the atom, making up the nucleus. • protons have a positive charge. • electrons have a negative charge. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. For each electron shell atom diagram, the element symbol is listed in the nucleus. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:
• electrons surround the nucleus. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. The negative charge of one electron balances the positive charge of one proton… 112 zeilen · 01.11.2021 · atomic no.

112 zeilen · 01.11.2021 · atomic no... Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Boron has 5 protons, 6 neutrons and 5 electrons: Protons, neutrons and electrons of all the elements: Beryllium has 4 protons, 5 neutrons and 4 electrons: • electrons surround the nucleus.

The mass of a proton is essentially the same as that of a neutron. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons... • electrons surround the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Carbon has 6 protons, 6 neutrons and 6 electrons

Beryllium has 4 protons, 5 neutrons and 4 electrons:. The negative charge of one electron balances the positive charge of one proton… Carbon has 6 protons, 6 neutrons and 6 electrons The atomic number of a sodium atom is 11 and its mass number is 23.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. • electrons have a negative charge. The negative charge of one electron balances the positive charge of one proton…

• electrons have a negative charge... • protons have a positive charge. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. The nucleus of an atom contains protons and neutrons. Beryllium has 4 protons, 5 neutrons and 4 electrons: • ˚e charge on the proton and electron are. Hydrogen has 1 proton, 0 neutron and 1 electron: Carbon has 6 protons, 6 neutrons and 6 electrons 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

The mass of a proton is 1840 times greater than the mass of an electron. The nucleus of an atom contains protons and neutrons. • protons have a positive charge. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The negative charge of one electron balances the positive charge of one proton… Beryllium has 4 protons, 5 neutrons and 4 electrons: The atomic number of a sodium atom is 11 and its mass number is 23.

The mass of a proton is 1840 times greater than the mass of an electron. The atomic number of a sodium atom is 11 and its mass number is 23. The nucleus of an atom contains protons and neutrons. The nucleus carries a positive electrical charge. The nucleus is positively charged since the proton is positively charged and the neutron is neutral.

The mass of a proton is essentially the same as that of a neutron. Protons and neutrons reside in the nucleus and are together called nucleons. Helium has 2 protons, 2 neutrons and 2 electrons: 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The mass of a proton is essentially the same as that of a neutron.. • electrons have a negative charge.

Protons, neutrons and electrons of all the elements: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 112 zeilen · 01.11.2021 · atomic no. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.

06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. The mass of a proton is essentially the same as that of a neutron. The mass of a proton is 1840 times greater than the mass of an electron. Carbon has 6 protons, 6 neutrons and 6 electrons. The nucleus is positively charged since the proton is positively charged and the neutron is neutral.

The mass of a proton is essentially the same as that of a neutron.. The nucleus of an atom contains protons and neutrons. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms.. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:. For that, we have electron shell diagrams. Lithium has 3 protons, 4 neutrons and 3 electrons: • ˚e charge on the proton and electron are. Hydrogen has 1 proton, 0 neutron and 1 electron: • electrons have a negative charge. The negative charge of one electron balances the positive charge of one proton… Protons, neutrons and electrons of all the elements: Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.. The mass of a proton is essentially the same as that of a neutron.
01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The mass of a proton is essentially the same as that of a neutron. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. The nucleus of an atom contains protons and neutrons. The mass of a proton is 1840 times greater than the mass of an electron.. Hydrogen has 1 proton, 0 neutron and 1 electron:

The nucleus carries a positive electrical charge. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. Calculate the number of protons, neutrons and electrons it contains.

The nucleus carries a positive electrical charge... Boron has 5 protons, 6 neutrons and 5 electrons: 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. The negative charge of one electron balances the positive charge of one proton…. The nucleus of an atom contains protons and neutrons.

As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.. .. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons.
• protons and neutrons are in the center of the atom, making up the nucleus.. The atomic number of a sodium atom is 11 and its mass number is 23. The mass of a proton is 1840 times greater than the mass of an electron. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. • ˚e charge on the proton and electron are.. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.

Beryllium has 4 protons, 5 neutrons and 4 electrons:. The nucleus carries a positive electrical charge. Boron has 5 protons, 6 neutrons and 5 electrons: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Carbon has 6 protons, 6 neutrons and 6 electrons The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 112 zeilen · 01.11.2021 · atomic no.

01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The nucleus of an atom contains protons and neutrons. Protons, neutrons and electrons of all the elements:

For each electron shell atom diagram, the element symbol is listed in the nucleus... • electrons have a negative charge. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. Protons and neutrons reside in the nucleus and are together called nucleons. Protons and neutrons reside in the nucleus and are together called nucleons.

• ˚e charge on the proton and electron are.. Boron has 5 protons, 6 neutrons and 5 electrons: 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. The nucleus carries a positive electrical charge... Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.

Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Protons and neutrons reside in the nucleus and are together called nucleons. The atomic number of a sodium atom is 11 and its mass number is 23. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Boron has 5 protons, 6 neutrons and 5 electrons: Carbon has 6 protons, 6 neutrons and 6 electrons For that, we have electron shell diagrams. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Calculate the number of protons, neutrons and electrons it contains. Lithium has 3 protons, 4 neutrons and 3 electrons: For that, we have electron shell diagrams.

• electrons surround the nucleus... 112 zeilen · 01.11.2021 · atomic no. Lithium has 3 protons, 4 neutrons and 3 electrons: The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The mass of a proton is 1840 times greater than the mass of an electron. For that, we have electron shell diagrams. The nucleus carries a positive electrical charge. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. The nucleus of an atom contains protons and neutrons. Protons, neutrons and electrons of all the elements:.. • protons have a positive charge.

The mass of a proton is essentially the same as that of a neutron. Protons, neutrons and electrons of all the elements: For each electron shell atom diagram, the element symbol is listed in the nucleus. • protons and neutrons are in the center of the atom, making up the nucleus. The nucleus of an atom contains protons and neutrons. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

The negative charge of one electron balances the positive charge of one proton… Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

Protons and neutrons reside in the nucleus and are together called nucleons. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The mass of a proton is essentially the same as that of a neutron. The nucleus carries a positive electrical charge. • protons and neutrons are in the center of the atom, making up the nucleus. • ˚e charge on the proton and electron are. The negative charge of one electron balances the positive charge of one proton….. The nucleus carries a positive electrical charge.

Protons, neutrons and electrons of all the elements:. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus... The mass of a proton is essentially the same as that of a neutron.

Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. • protons have a positive charge. The mass of a proton is essentially the same as that of a neutron. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Carbon has 6 protons, 6 neutrons and 6 electrons.. • ˚e charge on the proton and electron are.

The negative charge of one electron balances the positive charge of one proton….. • protons and neutrons are in the center of the atom, making up the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Carbon has 6 protons, 6 neutrons and 6 electrons 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. The negative charge of one electron balances the positive charge of one proton…

Hydrogen has 1 proton, 0 neutron and 1 electron: The nucleus carries a positive electrical charge. Beryllium has 4 protons, 5 neutrons and 4 electrons: The nucleus of an atom contains protons and neutrons. 112 zeilen · 01.11.2021 · atomic no. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons... 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms.

The nucleus carries a positive electrical charge.. Protons, neutrons and electrons of all the elements: 112 zeilen · 01.11.2021 · atomic no. • electrons have a negative charge. For that, we have electron shell diagrams.. Hydrogen has 1 proton, 0 neutron and 1 electron:

The mass of a proton is essentially the same as that of a neutron. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. • electrons surround the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. • protons have a positive charge. The atomic number of a sodium atom is 11 and its mass number is 23. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons and neutrons reside in the nucleus and are together called nucleons. The mass of a proton is essentially the same as that of a neutron. • protons and neutrons are in the center of the atom, making up the nucleus. Protons, neutrons and electrons of all the elements: 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

• ˚e charge on the proton and electron are. Carbon has 6 protons, 6 neutrons and 6 electrons Calculate the number of protons, neutrons and electrons it contains. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Beryllium has 4 protons, 5 neutrons and 4 electrons: Hydrogen has 1 proton, 0 neutron and 1 electron: 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. Protons, neutrons and electrons of all the elements:.. Hydrogen has 1 proton, 0 neutron and 1 electron:

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water... As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

Calculate the number of protons, neutrons and electrons it contains. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. • protons have a positive charge.
• protons have a positive charge. Carbon has 6 protons, 6 neutrons and 6 electrons The nucleus of an atom contains protons and neutrons. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons.
Boron has 5 protons, 6 neutrons and 5 electrons:. Boron has 5 protons, 6 neutrons and 5 electrons: 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. Beryllium has 4 protons, 5 neutrons and 4 electrons: For that, we have electron shell diagrams. 112 zeilen · 01.11.2021 · atomic no. Lithium has 3 protons, 4 neutrons and 3 electrons: The nucleus is positively charged since the proton is positively charged and the neutron is neutral.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
The atomic number of a sodium atom is 11 and its mass number is 23. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The nucleus of an atom contains protons and neutrons. The mass of a proton is essentially the same as that of a neutron.. Carbon has 6 protons, 6 neutrons and 6 electrons

The mass of a proton is 1840 times greater than the mass of an electron... The nucleus carries a positive electrical charge. Calculate the number of protons, neutrons and electrons it contains. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water... Carbon has 6 protons, 6 neutrons and 6 electrons Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. The mass of a proton is 1840 times greater than the mass of an electron... The nucleus carries a positive electrical charge.

The nucleus carries a positive electrical charge. The mass of a proton is 1840 times greater than the mass of an electron. Calculate the number of protons, neutrons and electrons it contains. Boron has 5 protons, 6 neutrons and 5 electrons:
/atom--illustration-713786859-5bdb6f7d46e0fb002d6db6df.jpg)
Hydrogen has 1 proton, 0 neutron and 1 electron:. • electrons have a negative charge. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons.. Lithium has 3 protons, 4 neutrons and 3 electrons:

20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms.. Protons and neutrons reside in the nucleus and are together called nucleons. The mass of a proton is 1840 times greater than the mass of an electron. Calculate the number of protons, neutrons and electrons it contains. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. For each electron shell atom diagram, the element symbol is listed in the nucleus. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

• electrons surround the nucleus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The atomic number of a sodium atom is 11 and its mass number is 23. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. Calculate the number of protons, neutrons and electrons it contains. • electrons surround the nucleus. Protons and neutrons reside in the nucleus and are together called nucleons. • protons and neutrons are in the center of the atom, making up the nucleus. Boron has 5 protons, 6 neutrons and 5 electrons: The nucleus of an atom contains protons and neutrons... For each electron shell atom diagram, the element symbol is listed in the nucleus.

• electrons have a negative charge. • electrons have a negative charge. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.. For that, we have electron shell diagrams.

06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons... • ˚e charge on the proton and electron are. Boron has 5 protons, 6 neutrons and 5 electrons: Protons, neutrons and electrons of all the elements: 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. For each electron shell atom diagram, the element symbol is listed in the nucleus.

For each electron shell atom diagram, the element symbol is listed in the nucleus. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. The mass of a proton is essentially the same as that of a neutron. Lithium has 3 protons, 4 neutrons and 3 electrons: The nucleus of an atom contains protons and neutrons. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:. 04.08.2021 · an atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons.

112 zeilen · 01.11.2021 · atomic no. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Hydrogen has 1 proton, 0 neutron and 1 electron: For that, we have electron shell diagrams. Protons and neutrons reside in the nucleus and are together called nucleons. Beryllium has 4 protons, 5 neutrons and 4 electrons: Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Boron has 5 protons, 6 neutrons and 5 electrons: 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Carbon has 6 protons, 6 neutrons and 6 electrons

The nucleus carries a positive electrical charge... • protons and neutrons are in the center of the atom, making up the nucleus. The atomic number of a sodium atom is 11 and its mass number is 23. • electrons have a negative charge. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. Hydrogen has 1 proton, 0 neutron and 1 electron: Helium has 2 protons, 2 neutrons and 2 electrons: 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles:

Protons and neutrons reside in the nucleus and are together called nucleons. 06.05.2019 · protons and neutrons are about the same size as each other and are much larger than electrons. The atomic number of a sodium atom is 11 and its mass number is 23. 01.09.2015 · 2.1 electrons, protons, neutrons, and atoms all matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: • electrons have a negative charge. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged... • electrons have a negative charge.

Calculate the number of protons, neutrons and electrons it contains.. • electrons have a negative charge. Beryllium has 4 protons, 5 neutrons and 4 electrons: Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. • protons and neutrons are in the center of the atom, making up the nucleus. Helium has 2 protons, 2 neutrons and 2 electrons:. Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

Boron has 5 protons, 6 neutrons and 5 electrons: Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. For that, we have electron shell diagrams. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Carbon has 6 protons, 6 neutrons and 6 electrons • electrons surround the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. The atomic number of a sodium atom is 11 and its mass number is 23. Helium has 2 protons, 2 neutrons and 2 electrons: The mass of a proton is 1840 times greater than the mass of an electron.

112 zeilen · 01.11.2021 · atomic no. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. For that, we have electron shell diagrams. Hydrogen has 1 proton, 0 neutron and 1 electron: 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Helium has 2 protons, 2 neutrons and 2 electrons: Helium has 2 protons, 2 neutrons and 2 electrons:

Calculate the number of protons, neutrons and electrons it contains. The atomic number of a sodium atom is 11 and its mass number is 23. • protons and neutrons are in the center of the atom, making up the nucleus. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms.. Calculate the number of protons, neutrons and electrons it contains.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms... As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Protons, neutrons, and electrons key concepts • atoms are made of extremely tiny particles called protons, neutrons, and electrons. Helium has 2 protons, 2 neutrons and 2 electrons: Beryllium has 4 protons, 5 neutrons and 4 electrons: For that, we have electron shell diagrams. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. • electrons surround the nucleus. The mass of a proton is 1840 times greater than the mass of an electron. Calculate the number of protons, neutrons and electrons it contains. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. • electrons have a negative charge.

Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged... Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. Beryllium has 4 protons, 5 neutrons and 4 electrons:. As summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged.

23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: Protons, neutrons, and electrons.as summarized in table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The mass of a proton is essentially the same as that of a neutron. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. 20.07.2016 · it's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Helium has 2 protons, 2 neutrons and 2 electrons: Carbon has 6 protons, 6 neutrons and 6 electrons

The nucleus is positively charged since the proton is positively charged and the neutron is neutral. Beryllium has 4 protons, 5 neutrons and 4 electrons:

The atomic number of a sodium atom is 11 and its mass number is 23. Hydrogen has 1 proton, 0 neutron and 1 electron: For that, we have electron shell diagrams. The nucleus of an atom contains protons and neutrons. • electrons surround the nucleus. 23.09.2019 · 2.1 electrons, protons, neutrons, and atoms all matter that we are familiar with, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: The mass of a proton is 1840 times greater than the mass of an electron. The atomic number of a sodium atom is 11 and its mass number is 23. • protons and neutrons are in the center of the atom, making up the nucleus. The negative charge of one electron balances the positive charge of one proton…

For that, we have electron shell diagrams. The negative charge of one electron balances the positive charge of one proton… For that, we have electron shell diagrams. • electrons have a negative charge.
